Urethral strictures in men, Windsor, Berkshire & Slough
INTRODUCTION
Urethral stricture disease is relatively common in men with most patients acquiring the disease due to injury or infection. The most common aetiology for stricture is iatrogenic injury due to previous instrumentation such as catheterisation [1].
Although some patients are able to relate a definitive history of prior instrumentation, injury or infection, the aetiology of the stricture often remains unknown. Most patients present with chronic voiding symptoms, but acute urinary obstruction can occur without significant warning, requiring emergency urethral dilation or insertion of a supra-pubic catheter.
The pre-treatment evaluation aims to identify the location, length and severity of the stricture and guides the choice of treatment.
ANATOMY AND PATHOPHYSIOLOGY
The urethra conveys urine from the bladder to the exterior of the body. Pelvic fractures can cause distraction defects in the posterior urethra, whereas blunt perineal trauma injures the bulbar urethra. The male urethra is divided into two major segments: the anterior urethra and the posterior urethra (Figure 1).
- The anterior urethra begins at the meatus of the penis and includes the fossa navicularis, the pendulous urethra, and the bulbar urethra. The suspensory ligament of the penis delineates the pendulous and bulbar urethra. The anterior urethra consists of an endothelial layer that is surrounded by the urethral spongiosum. The spongiosum is concentrically located around the urethra in the distal pendulous urethra. In the bulbar urethra, it becomes eccentrically located with a larger component on the ventral surface.
- The posterior urethra includes the membranous urethra, prostatic urethra and the bladder neck; the spongiosum is absent.
The male urethra is supplied proximally by the bulbar arteries which are branches of the penile artery, whereas the distal urethra is perfused by retrograde flow from the dorsal penile artery.
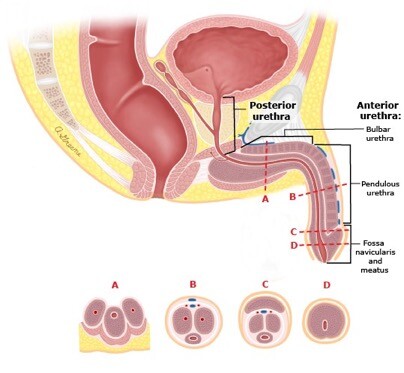
Figure 1. Anatomy of the male urethra
INDICATIONS FOR TREATMENT
Patients may present with a variety of symptoms including obstructive voiding symptoms, acute urinary
retention, recurrent urinary tract infection, or more rarely, hydronephrosis, urethral fistula or periurethral abscess. The commonest presenting symptoms include weak stream (49%) and incomplete bladder emptying (27%) [2]. However, up to 21% do not present with voiding symptoms but may complain of spraying of urinary stream (13%) and dysuria or painful voiding (10%). Furthermore, up to 10% of patients may have no significant symptoms.
Treatment is indicated in patients with urethral stricture associated with severe voiding symptoms, acute urinary retention, bladder stones, a high post-void residual or recurrent urinary tract infection.
APPROACH TO TREATMENT
The approach to the treatment of urethral stricture disease depends upon the location, aetiology and length of the stricture.
Treatment options for urethral stricture include several minimally-invasive therapies (dilation, endoscopic urethrotomy), urinary diversion procedures (suprapubic catheter, perineal urethrostomy), and surgical reconstruction of the urethra using flaps or grafts. No single procedure is appropriate to manage all strictures, and multiple techniques may be used in the same patient during the course of treatment to manage recurrent stricture. Urethral dilation and urethrotomy, which are minimally-invasive techniques, continue to be the most commonly- employed initial treatments [3]. Endoscopic urethrotomy is quick, widely available and safe. Long-term success rates are around 50% but are improved for strictures <1 cm, those located in the mid-bulbar urethra, and those with at least 5 mm of preserved lumen. Patients with severe strictures and those that recur early may need
surgical reconstruction [4].
Urethral reconstruction involves varying degrees of stricture incision or excision (urethroplasty) with or without augmentation using a flap or graft for reconstruction. The potential effects of the chosen surgical technique on erectile function are important to consider. Penile elongation during erection requires that surgical repairs of the penile urethra be elastic; local flaps in this location are superior to grafts. Similarly, primary anastomotic urethroplasty, which is useful for repairing the bulbar urethra, can result in chordee with erection when used in the penile urethra.
PREPARATION
Prior to instrumentation or surgery, sterile urine should be verified. Prior to treatment, the anatomic site, length, and severity of the narrowed segment must be defined. A retrograde urethrogram or urethroscopy may be helpful to help define the extent of urethral mucosal involvement.
Antibiotics — Antibiotic prophylaxis is recommended for all procedures involving cystourethroscopy with manipulation and surgical treatment of urethral strictures to minimize the potential for infection [5,6]. Patients with colonization or symptomatic urinary tract infection should be treated with broad-spectrum oral
antibiotics, and instrumentation or surgery should be delayed, if possible, until the urine is clear. Positive urine cultures are associated with a higher rate of complications [7]. However, urinary tract infection sometimes cannot be completely cleared until urethral obstruction is treated and the bladder completely decompressed. For these patients, antibiotic therapy is continued following instrumentation.
The initial diagnosis of urethral stricture is often made with a retrograde urethrogram (RUG) or Urethroscopy.
MINIMALLY-INVASIVE THERAPIES
Several minimally-invasive techniques are available for the initial management of urethral strictures including dilation with balloons or serial axial dilators (filiforms and followers), cold knife incision and incision with Holmium laser which is now becoming the Gold Standard.
Techniques
Urethral dilation
Axial dilation of urethral strictures is most commonly performed using filiforms and followers under endoscopic control. Urethral dilation is an appropriate initial treatment when the history and imaging evaluation have found
a stricture less than 2 cm with no associated spongiofibrosis, and no complex features such as a fistula or a diverticulum.
Following successful dilation, the patient can be taught self-calibration with a soft 14 to 16 French catheter to maintain urethral patency. The patient typically performs self-calibration once a day for a week after the initial dilation and then at decreasing intervals over time. Few patients are compliant with self calibration in the long term.
Endoscopic urethrotomy
Direct vision internal urethrotomy (DVIU) may be used after after axial dilation for the initial treatment of simple strictures or as a primary procedure. Urethrotomy is aided by a guidewire placed through the stricture. The urethrotomy incision is usually made at the 12 o’clock with a cold-knife urethrotome. However, more recently the incision can be made more safely and effectively with a Holmium laser.
Urethrotomy is especially well-suited for tight, short strictures (<2 cm) of the bulbar urethra. Longer strictures, strictures involving the pendulous urethra, or strictures associated with significant spongiofibrosis are associated with higher failure rates [8].
- Strictures <1 cm in length respond well to minimally-invasive therapies [9,11]. In one study, strictures with a length <1 cm had a recurrence rate of 27 percent compared with 50 percent for those ≥1 cm in length [18].
- Bulbar strictures with a preserved luminal diameter >15 French (5 mm) had the best overall outcomes in a retrospective review of 224 patients who underwent internal urethrotomy [10].
- The mid-bulbar urethra appears to respond the best to dilation, incision, or ablation with laser. The bulbar urethra has an abundant corpus spongiosum, which helps to decrease scarring. Direct vision internal urethrotomy (DVIU) is most likely to be successful in this segment of the urethra.
- Recurrence rates are higher for treatment of strictures involving the pendulous urethra, or strictures associated
What happens during the procedure?
A full general anaesthetic is normally used and you will be given an injection of antibiotics before the procedure after you have been checked for any allergies. The surgeon will insert a telescope into the penis through the water pipe (urethra). The urethra or urethral opening is then stretched using metal (Figure 2) or plastic dilators, after inserting local anaesthetic jelly to numb and lubricate the passage. The alternative is to cut any narrowing using a special internal knife or a laser fibre (Figure 3). All the cutting takes place internally and there are no incisions or stitches. Most patients need a bladder catheter for 24 hours afterwards.
The average hospital stay is one night, especially if a bladder catheter has been put in. You may need to subsequently learn a technique of intermittent self-dilatation; this involves passing a plastic tube down the urethra, usually once a week, to prevent future tightening of the urethra.
You will often have some bleeding around the catheter because the cut is made in the water pipe (urethra) that surrounds the catheter. This usually lasts for a short period unless the surgeon has made multiple or deep cuts. A pad will often be secured around the end of the penis to collect any blood which seeps out around the
catheter; this is usually removed the next day.
Once the catheter has been removed, you should be able to pass urine with an improved flow. In the early stages, this can be painful and blood-stained but, provided you drink plenty of fluid, this will gradually settle over a few days. Once the initial discomfort settles, you will be asked to do a flow rate test to see how fast you pass urine; this will be used as a “baseline” to compare with future measurements.
Are there any side-effects?
Most procedures have possible side-effects. But, although the complications listed below are well-recognised, most patients do not suffer any problems.
Common (greater than 1 in 10)
Mild burning or bleeding on passing urine for a short period after the operation.
Need for self catheterisation to keep the narrowing from closing down again.
Recurrence of narrowing needing further procedures or repeat incision.
Occasional (between 1 in 10 and 1 in 50)
Infection of the bladder needing antibiotics.
Permission for telescopic removal or biopsy of any bladder abnormality or stone, if found.
Rare (less than 1 in 50)
Decrease in the quality of erections.
Leakage of urine usually short term

Figure 2. Cluttons dilators for urethral strictures
Figure 3. Urethral stricture treated with Holmium laser fibre
REFERENCES
- Lumen N, Hoebeke P, Willemsen P, et al. Aetiology of urethral stricture disease in the 21st century. J Urol 2009; 182:983.
- Nuss GR, Granieri MA, Zhao LC, et al. Presenting symptoms of anterior urethral stricture disease: a disease specific, patient reported questionnaire to measure outcomes. J Urol 2012; 187:559.
- Bullock TL, Brandes SB. Adult anterior urethral strictures: a national practice patterns survey of board certified urologists in the United States. J Urol 2007; 177:685.
- Barbagli G, Palminteri E, Lazzeri M, et al. Long-term outcome of urethroplasty after failed urethrotomy versus primary repair. J Urol 2001; 165:1918.
- Barbagli G, Palminteri E, Guazzoni G, et al. Bulbar urethroplasty using buccal mucosa grafts placed on the ventral, dorsal or lateral surface of the urethra: are results affected by the surgical technique? J Urol
2005; 174:955.
- Morey AF, Lin HC, DeRosa CA, Griffith BC. Fossa navicularis reconstruction: impact of stricture length on outcomes and assessment of extended meatotomy (first stage Johanson) maneuver. J Urol 2007;
177:184.
- Steenkamp JW, Heyns CF, de Kock ML. Outpatient treatment for male urethral strictures–dilatation versus internal urethrotomy. S Afr J Surg 1997; 35:125.
- Stone AR, Randall JR, Shorrock K, et al. Optical urethrotomy–a 3-year experience. Br J Urol 1983;
55:701.
- Albers P, Fichtner J, Brühl P, Müller SC. Long-term results of internal urethrotomy. J Urol 1996;
156:1611.
- Pansadoro V, Emiliozzi P. Internal urethrotomy in the management of anterior urethral strictures: long- term followup. J Urol 1996; 156:73.
- Rourke KF, Jordan GH. Primary urethral reconstruction: the cost minimized approach to the bulbous urethral stricture. J Urol 2005; 173:1206.










